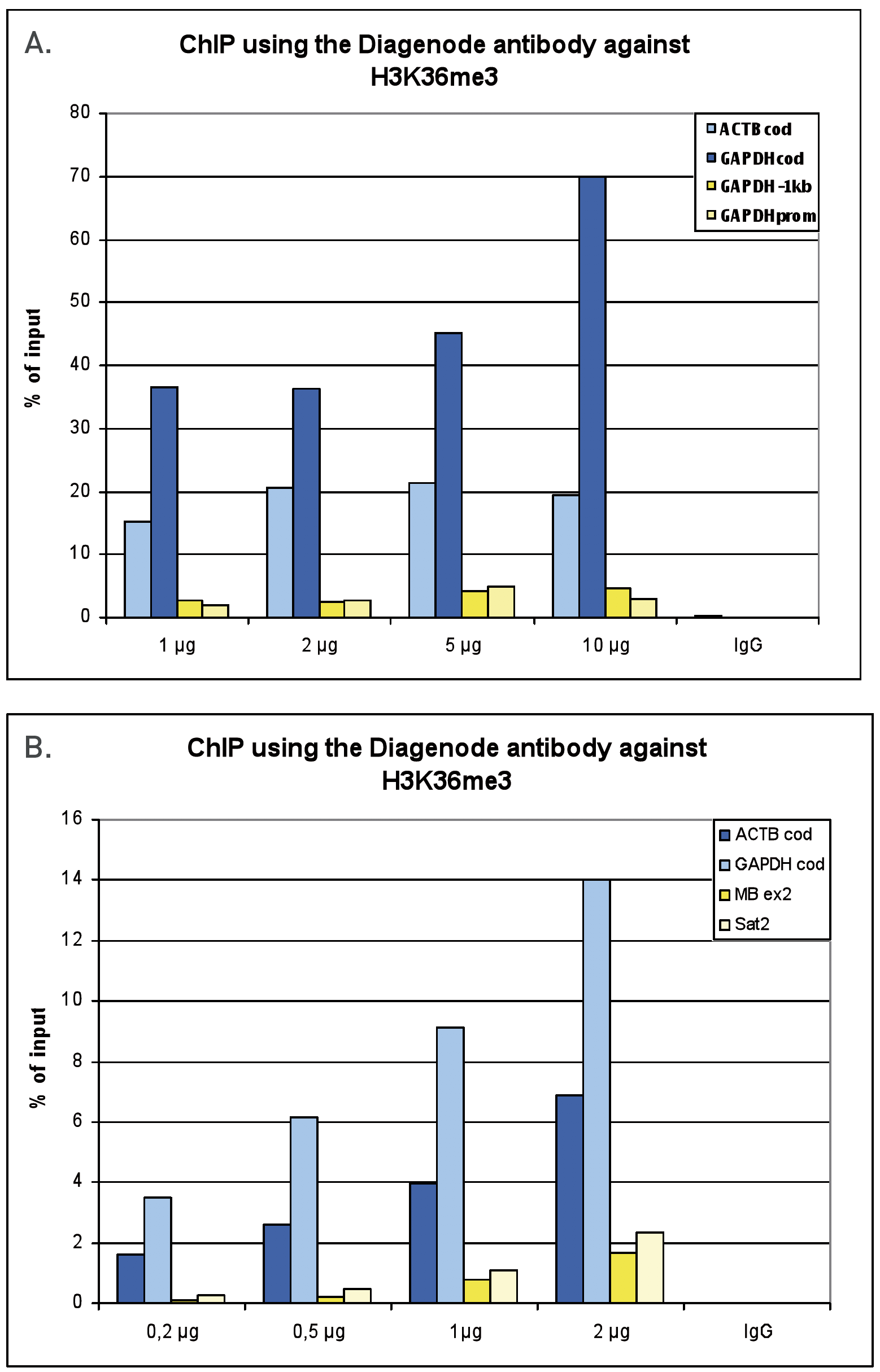
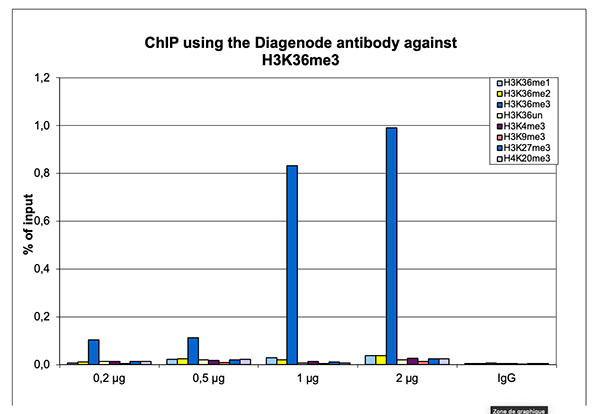
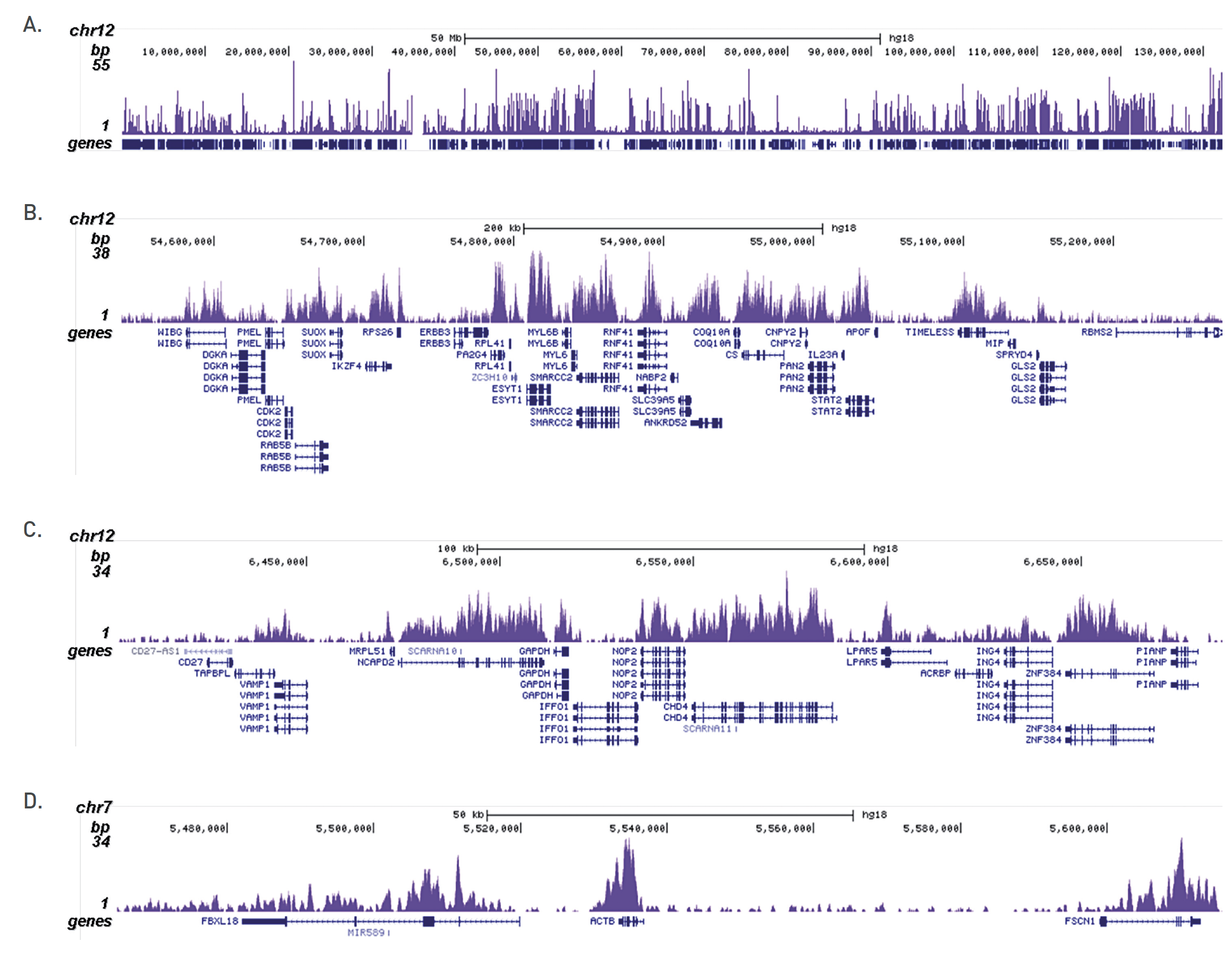
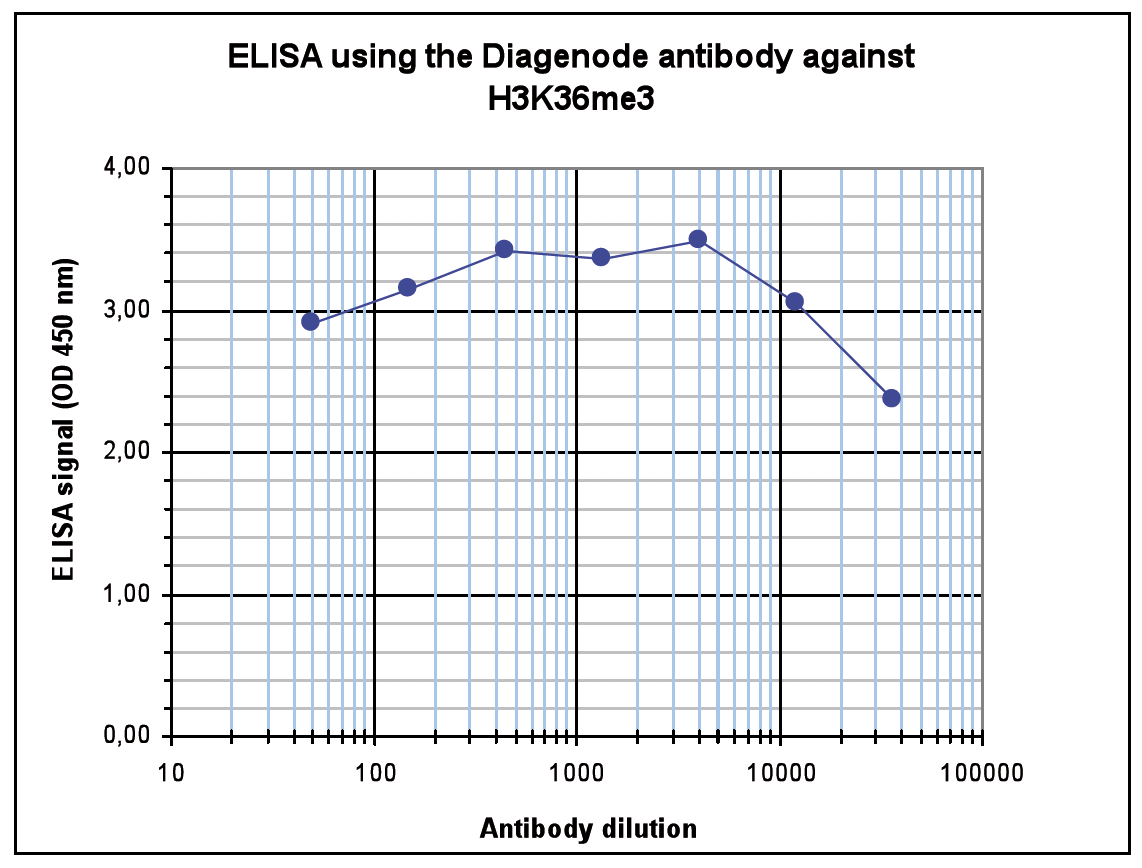
Figure 1. ChIP results obtained with the Diagenode antibody directed against H3K36me3
Figure 1A ChIP assays were performed using human HeLa cells, the Diagenode antibody against H3K36me3 (Cat. No. C15410192) and optimized PCR primer pairs for qPCR. ChIP was performed with the “Auto Histone ChIP-seq” kit (Cat. No. C01010022) on the IP-Star automated system, using sheared chromatin from 1,000,000 cells. A titration consisting of 1, 2, 5 and 10 μg of antibody per ChIP experiment was analyzed. IgG (2 μg/IP) was used as a negative IP control. Quantitative PCR was performed with primers for the coding region of the active GAPDH and ACTB genes, used as positive controls, and for the promoter and a region located 1 kb upstream of the promoter of the GAPDH gene, used as negative controls.
Figure 1B ChIP assays were performed using human K562 cells, the Diagenode antibody against H3K36me3 (Cat. No. C15410192) and optimized PCR primer pairs for qPCR. ChIP was performed with the “iDeal ChIP-seq” kit (Cat. No. C01010051), using sheared chromatin from 100,000 cells. A titration consisting of 0.2, 0.5, 1 and 2 μg of antibody per ChIP experiment was analyzed. IgG (1 μg/IP) was used as a negative IP control. Quantitative PCR was performed with primers for the coding region of the active GAPDH and ACTB genes, used as positive controls, and for the coding region of the inactive MB gene and the Sat satellite repeat, used as negative controls. Figure 1 shows the recovery, expressed as a % of input (the relative amount of immunoprecipitated DNA compared to input DNA after qPCR analysis).